Abstract
Introduction:
Genetic rearrangements in both the MYC gene and anti-apoptotic genes such as BCL2 or BCL6 in diffuse large B-cell lymphoma (DLBCL) represent a sub-type of lymphoma, termed "double-hit lymphoma" (DHL) that is associated with poor outcomes and a poor response to standard chemoimmunotherapy regimens such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) (Petrich et al, Blood, 2014). The incidence of DHL is low at approximately 5-10% of DLBCL presentations. Alternatively, DLBCL cases featuring dual expression of both MYC and BCL-2 and/or BCL-6 (as detected by immunohistochemistry [IHC]) occur more frequently with increasing evidence to suggest this lymphoma-subtype, termed "double-expressor lymphoma" (DEL) may also respond poorly to induction therapy with R-CHOP (Green et al, JCO, 2012). Studies examining outcomes for DEL are often limited by an inability to separate DHL from DEL or lack a direct comparison to R-CHOP and, as a result, optimal induction therapy for DEL remains an unanswered question.
Methods
We performed a retrospective review of outcomes in DEL according to initial treatment regimen (R-CHOP vs intensive therapy) for patients diagnosed from 2013-2016 at the University of Wisconsin. We excluded patients lacking IHC or FISH data for MYC or BCL-2, those receiving a non-anthracycline containing regimen, those with concurrent central nervous system presentation, and those with post-transplant lymphoproliferative disorder. Any patients meeting criteria for DHL (i.e. dual gene rearrangements) were coded as DHL and not coded as DEL regardless of MYC, BCL-2, or BCL-6 expression. Intensive therapy included dose adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R), or rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone (R-hyper-CVAD).
Results
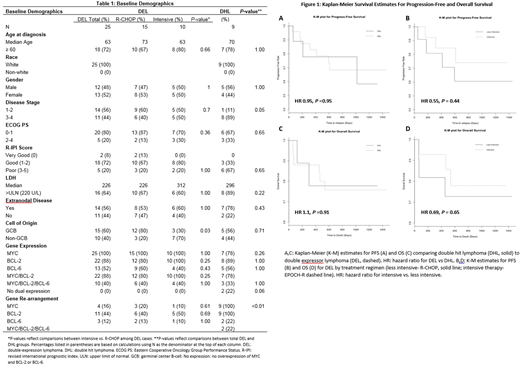
From 200 cases of DLBCL, we identified 31 (16%) cases of DEL and 11 (6%) cases of DHL. Twenty-five cases of DEL and 9 cases of DHL were eligible for inclusion in our study. Characteristics were similar between the DHL and DEL cohort, although there were more cases of early stage disease in the DEL cohort (56% vs 11%, P=0.05).
For DEL, the median age for the R-CHOP cohort was older compared to the intensive cohort (table 1, 73 vs 63), but both groups had a similar percentage of patients aged >60 years (67 vs 80%, P=0.66). Otherwise, demographics including performance status, stage, and revised international prognostic index score were well balanced between the two treatment groups with the exception of more cases of germinal center B-cell of origin (GCB) within the R-CHOP group (80 v 30%, P=0.03).
Complete response rates to induction regimens for DEL were similar between the R-CHOP and intensive group (76 vs 80%, P=0.65). Median progression free survival (PFS) was extended in the intensive cohort although 2 year PFS rates were similar between the two groups (40 vs 50%, P=0.70). Two-year overall survival rates were also similar (80 vs 70%, P=0.65). Kaplan-Meier survival curves (figure 1) illustrate a potential benefit for intensive induction therapy, however this is not statistically significant (hazard ratio 0.69 P=0.65).
Compared to patients with DEL, patients with DHL achieved similar complete response rates to induction therapy (76% vs 67%, P=0.67) despite exclusive use of DA-EPOCH-R for all DHL patients. There were more consolidative autologous stem cell transplants (auto-SCT) performed in the DHL group (33% vs 8%, P=0.1). PFS and OS were also similar between DHL and DEL sub-groups (HR 1.09, P=0.91)
Conclusions
Our study is amongst the first to compare R-CHOP to more intensive regimens for DEL while also separating DEL from DHL. Our current sample size limits our ability to draw definitive conclusions regarding outcomes by treatment regimen; however, despite lack of statistical significance, there is a trend toward intensive regimens improving PFS and OS in DEL. Our study is also amongst the first to directly compare outcomes for DEL and DHL. Survival for DEL was similar to DHL and may reflect improvements in outcomes for DHL when using intensive regimens or may be related to the increased use of consolidative auto-SCT for DHL. Our sample is part of a planned larger multi-center analysis and an increased sample size may build on the improved survival trend demonstrated for intensive regimens vs R-CHOP observed in our analysis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.